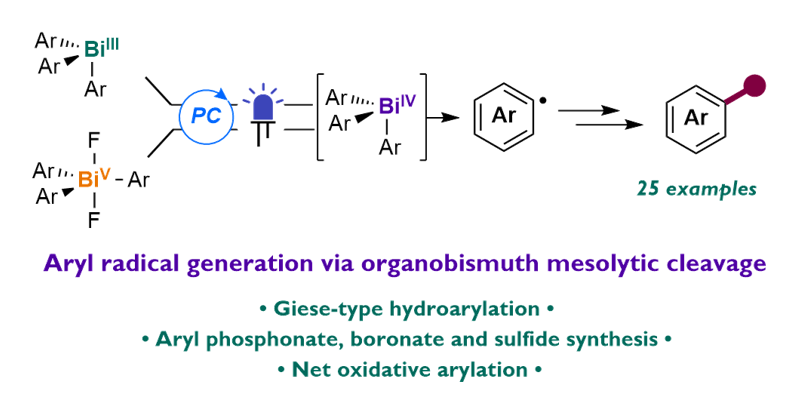
56. Organobismuth Compounds as Aryl Radical Precursors via Light-Driven Single-Electron Transfer
Nicholas D. Chiappini, Eric P. Geunes, Ethan T. Bodak, Robert R. Knowles
ACS Catal. 2024, 14, 2664–2670. DOI: 10.1021/acscatal.3c05598
https://pubs.acs.org/doi/10.1021/acscatal.3c05598
Abstract: A light-driven method for the generation of aryl radicals from triarylbismuth(III) and (V) reagents is described. Aryl radical generation is proposed to occur through the ligand-assisted mesolytic cleavage of an organobismuth(IV) intermediate generated from either oxidation of Bi(III) or reduction of Bi(V). This mode of aryl radical generation is demonstrated to be compatible with a range of bimolecular radical arylations, including hydroarylation of electron-deficient olefins and arylation of diboronates, disulfides, sulfonyl cyanides, phosphites, and isocyanides. The intermediacy of an aryl radical is supported by radical trapping and radical clock experiments, and Bi(IV)−aryl mesolysis is supported computationally.