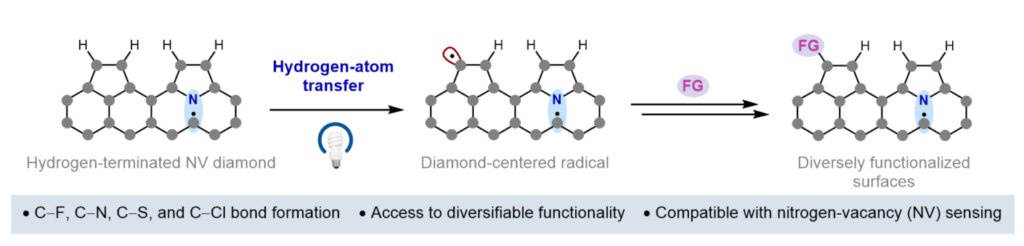
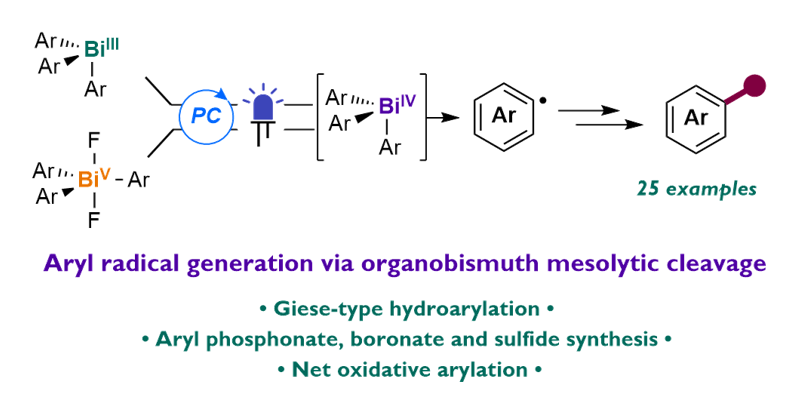
RESEARCH
Our lab is interested in addressing unsolved problems in synthetic organic chemistry and asymmetric catalysis. Key to our approach is a focus on strategies for the generation and downstream reactivity of radical intermediates using light-driven methods. One area of recent focus has been on the development of proton-coupled electron transfer (PCET) reactions. PCET is an unconventional redox process in which an electron and proton are exchanged together in a concerted elementary step. We have demonstrated that PCET can serve as a general mechanism for homolytic bond activation that allows for the generation of radical intermediates from the strong bonds found in numerous common organic functional groups. Another active research area in our group has been the development of out-of-equilibrium transformations driven by excited-state redox events. By using light to generate high-energy intermediates, reactions can proceed across two distinct potential surfaces, allowing for the formation of unconventional product distributions. We have used this platform to achieve challenging olefin hydrofunctionalization reactivities, unimolecular contra-thermodynamic isomerization reactions, and methods for light-driven depolymerization which cannot be achieved using conventional ground-state methods.