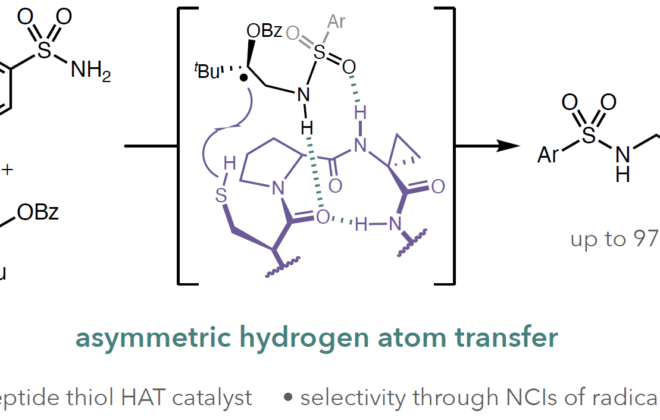
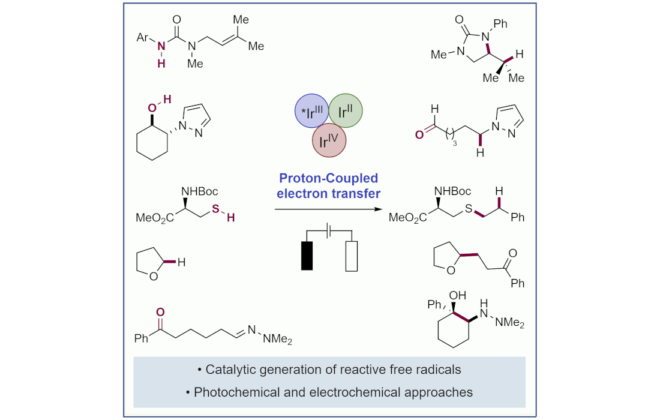
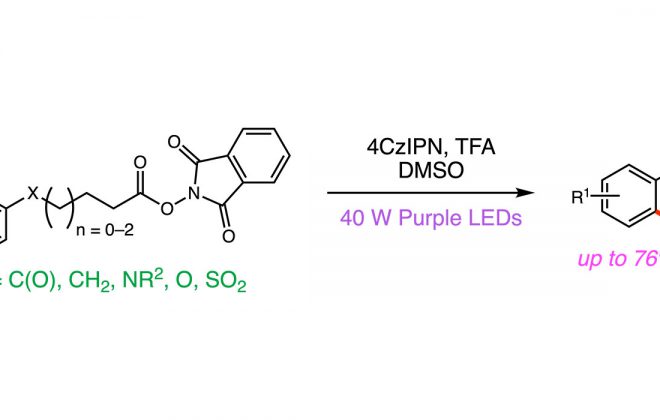
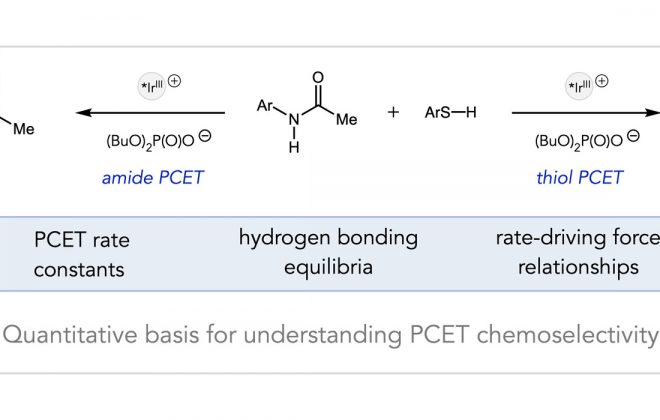
33. Catalytic Hydroetherification of Unactivated Alkenes Enabled by Proton‐Coupled Electron Transfer
Elaine Tsui, Anthony J. Metrano, Yuto Tsuchiya, Robert R. Knowles:
Angew. Chem., Int. Ed. 2020, 59, 11845–11849. DOI: 10.1002/anie.202003959
https://onlinelibrary.wiley.com/doi/10.1002/anie.202003959
ABSTRACT: We report a catalytic, light‐driven protocol for the intramolecular hydroetherification of unactivated alkenols to furnish cyclic ether products. These reactions occur under visible light irradiation in the presence of an Ir(III)‐based photoredox catalyst, a Brønsted base catalyst, and a hydrogen atom transfer co‐catalyst. Reactive alkoxy radicals are proposed as key intermediates, generated via the direct homolytic activation of alcohol O–H bonds through a proton‐coupled electron transfer mechanism. This method exhibits a broad substrate scope and high functional group tolerance, and it accommodates a diverse range of alkene substitution patterns. Results demonstrating the extension of this catalytic system to carboetherification reactions are also presented.