32. Enantioselective Hydroamination of Alkenes with Sulfonamides Enabled by Proton-Coupled Electron Transfer
Casey B. Roos, Joachim Demaerel, David E. Graff, Robert R. Knowles:
J. Am. Chem. Soc. 2020, 142, 5974–5979. DOI: 10.1021/jacs.0c01332
https://pubs.acs.org/doi/10.1021/jacs.0c01332
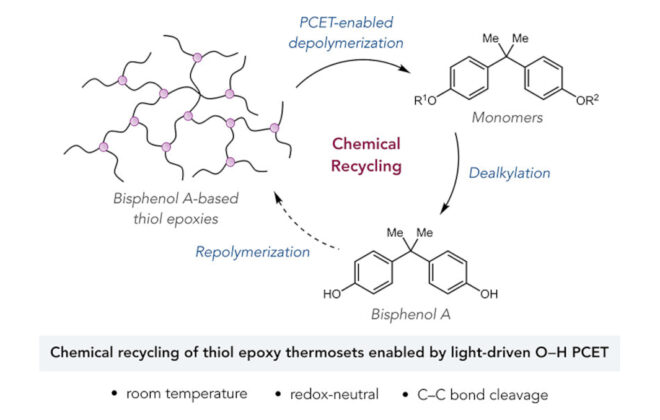
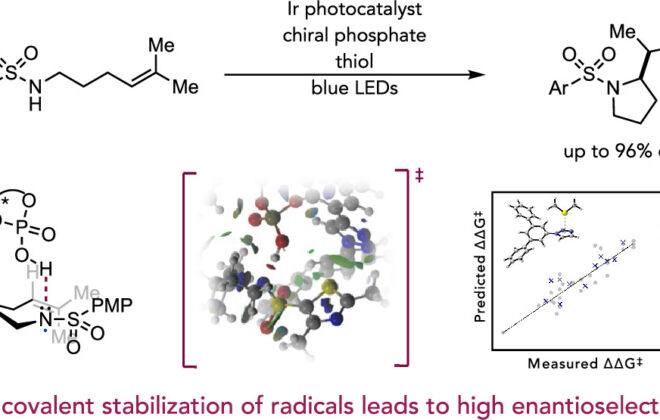
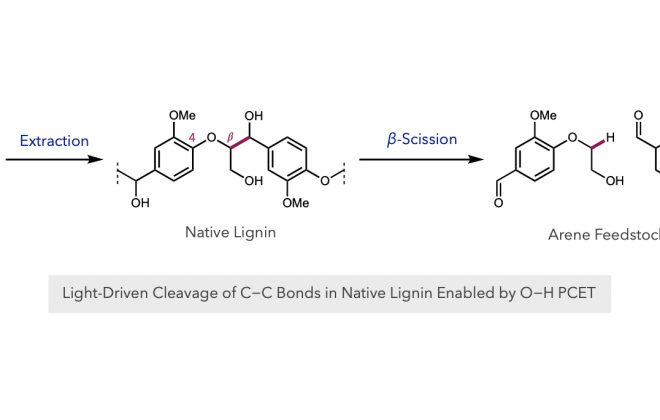
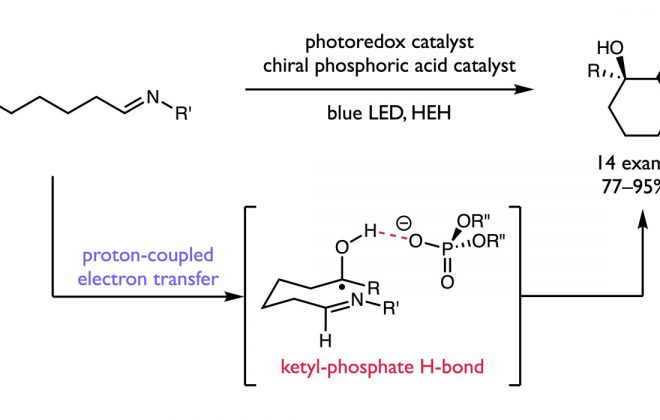
ABSTRACT: An enantioselective, radical-based method for the intramolecular hydroamination of alkenes with sulfonamides is reported. These reactions are proposed to proceed via N-centered radicals formed by proton-coupled electron transfer (PCET) activation of sulfonamide N–H bonds. Non-covalent interactions between the neutral sulfonamidyl radical and a chiral phosphoric acid generated in the PCET event are hypothesized to serve as the basis for asymmetric induction in a subsequent C–N bond forming step, achieving selectivities of up to 98:2 er. These results offer further support for the ability of non-covalent interactions to enforce stereoselectivity in reactions of transient and highly reactive open-shell intermediates.