47. Noncovalent Stabilization of Radical Intermediates in the Enantioselective Hydroamination of Alkenes with Sulfonamides
Eve Y. Xu, Jacob Werth, Casey B. Roos, Andrew J. Bendelsmith, Matthew S. Sigman, Robert R. Knowles
J. Am. Chem. Soc. 2022, 144, 18948–18958. DOI: 10.1021/jacs.2c07099
https://pubs.acs.org/doi/10.1021/jacs.2c07099
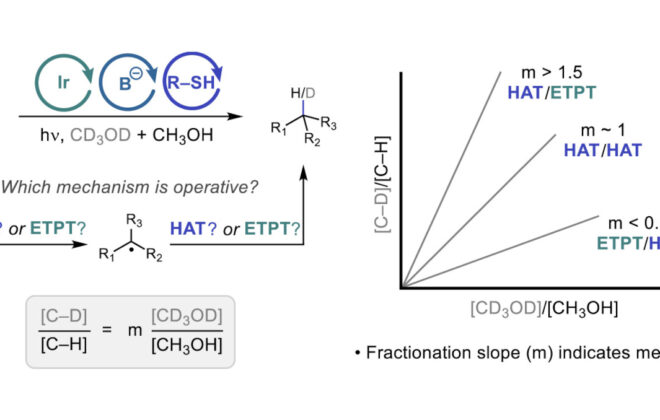
ABSTRACT: Noncovalent interactions (NCIs) are critical elements of molecular recognition in a wide variety of chemical contexts. While NCIs have been studied extensively for closed-shell molecules and ions, very little is understood about the structures and properties of NCIs involving free radical intermediates. In this report, we describe a detailed mechanistic study of the enantioselective radical hydroamination of alkenes with sulfonamides and present evidence suggesting that the basis for asymmetric induction in this process arises from attractive NCIs between a neutral sulfonamidyl radical intermediate and a chiral phosphoric acid (CPA). We describe experimental, computational, and data science-based evidence that identifies the specific radical NCIs that form the basis for the enantioselectivity. Kinetic studies support that C–N bond formation determines the enantioselectivity. Density functional theory investigations revealed the importance of both strong H-bonding between the CPA and the N-centered radical and a network of aryl-based NCIs that serve to stabilize the favored diastereomeric transition state. The contributions of these specific aryl-based NCIs to the selectivity were further confirmed through multivariate linear regression analysis by comparing the measured enantioselectivity to computed descriptors. These results highlight the power of NCIs to enable high levels of enantioselectivity in reactions involving uncharged open-shell intermediates and expand our understanding of radical–molecule interactions.