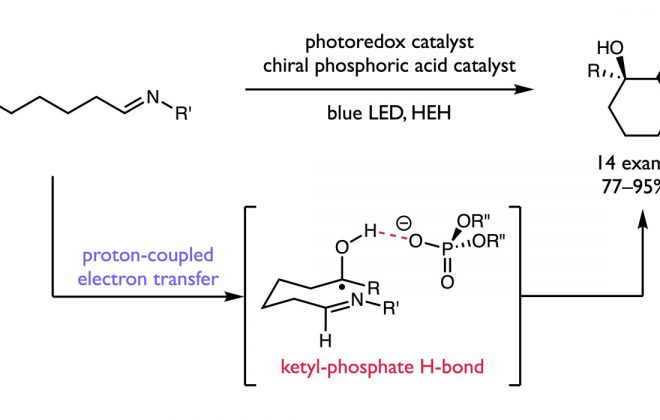
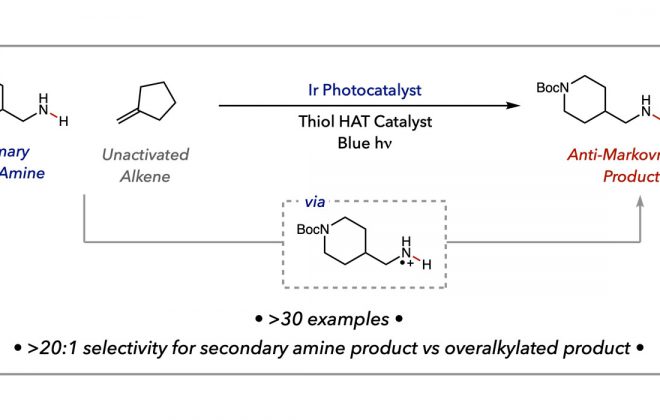
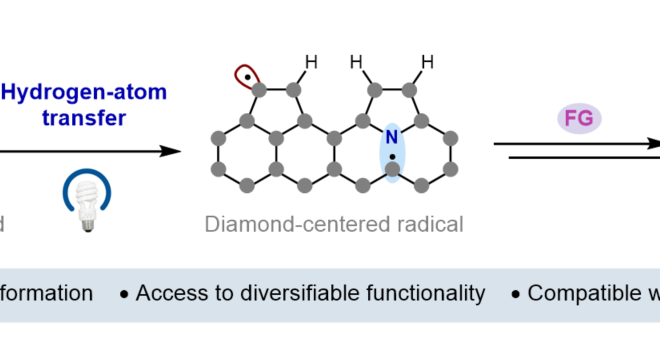
52. Isotopic Fractionation as a Mechanistic Probe in Light-Driven C–H Bond Exchange Reactions
Guanqi Qiu, Chi-Li Ni, and Robert R. Knowles
J. Am. Chem. Soc. 2023, 145 11537–11543. DOI: 10.1021/jacs.2c11212
https://pubs.acs.org/doi/10.1021/jacs.2c11212
ABSTRACT: Here, we report a diagnostic framework for elucidating the mechanisms of photoredox-based hydrogen isotope exchange (HIE) reactions based on hydrogen/deuterium (H/D) fractionation. Traditional thermal HIE methods generally proceed by reversible bond cleavage and bond reformation steps that share a common transition state. However, bond cleavage and bond reformation in light-driven HIE reactions can proceed via multiple, non-degenerate sets of elementary steps, complicating both mechanistic analysis and attendant optimization efforts. Building on classical treatments of equilibrium isotope effects, the fractionation method presented here extracts information regarding the nature of the key bond-forming and bond-breaking steps by comparing the extent of deuterium incorporation into an exchangeable C–H bond in the substrate relative to the H/D isotopic ratio of a solvent reservoir. We show that the extent of fractionation is sensitive to the mechanism of the exchange process and provides a means to distinguish between degenerate and non-degenerate mechanisms for isotopic exchange. In model systems, the mechanisms implied by the fractionation method align with those predicted by thermochemical considerations. We then employed the method to study HIE reactions whose mechanisms are ambiguous on thermodynamic grounds.