22. N–H Bond Formation in a Manganese(V) Nitride Yields Ammonia by Light-Driven Proton-Coupled Electron Transfer
Dian Wang, Florian Loose, Paul J. Chirik, Robert R. Knowles:
https://pubs.acs.org/doi/10.1021/jacs.8b12957
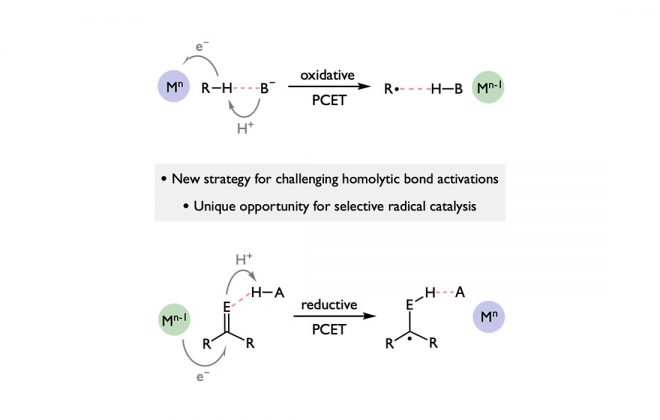
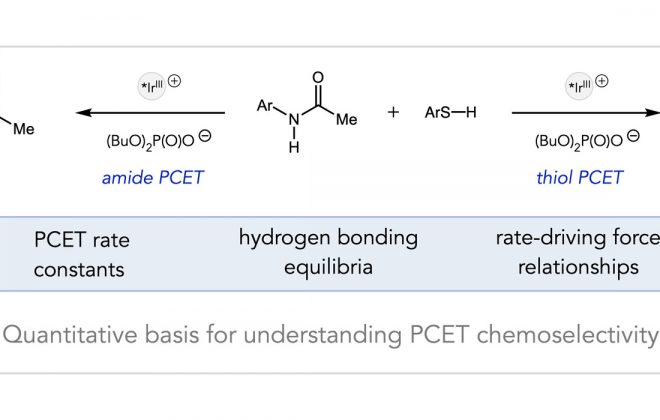
ABSTRACT: A method for the reduction of a manganese nitride to ammonia is reported, where light-driven proton-coupled electron transfer enables the formation of weak N—H bonds. Photoreduction of (saltBu)MnVN to ammonia and a Mn(II) complex has been accomplished using 9,10-dihydroacridine and a combination of an appropriately matched photoredox catalyst and weak Brønsted acid. Acid-reductant pairs with effective bond dissociation free energies between 35 and 46 kcal/mol exhibited high efficiencies. This light-driven method may provide a blueprint for new approaches to catalytic homogeneous ammonia synthesis under ambient conditions.
Categories