60. Intermolecular Anti-Markovnikov Hydroamination of Alkenes with Sulfonamides, Sulfamides, and Sulfamates
Angela Lin, Mathis J. Karrasch, Qiaolin Yan, Jacob M. Ganley, Benjamin G. Hejna, Robert R. Knowles
ACS Catal.. 2024, 14, 13098–13104. DOI: 10.1021/acscatal.4c03960
https://pubs.acs.org/doi/10.1021/acscatal.4c03960
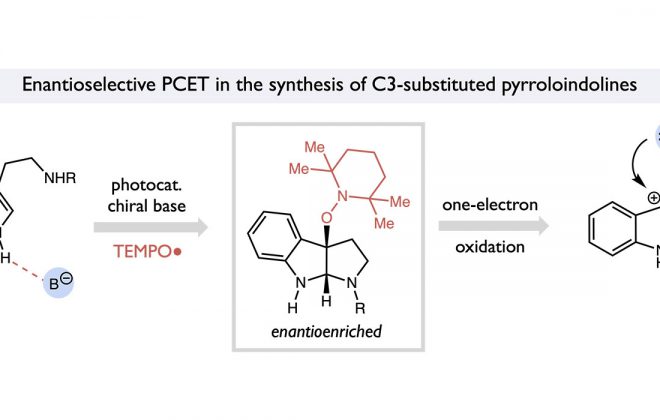
Abstract: A general method for the light-driven intermolecular anti-Markovnikov hydroamination of alkenes with primary sulfonamides, sulfamides, and sulfamates is presented. The reaction is mediated by a ternary catalyst system composed of an iridium(III) chromophore, a fluorinated alkoxide base, and a thiol H-atom donor. We hypothesize that the reactions proceed via a proton-coupled electron transfer (PCET) mechanism wherein implementation of the alkoxide base imparts additional thermochemical driving force for the homolytic activation of strong N–H bonds that were previously inaccessible using this methodology. This furnishes electrophilic N-centered radicals that subsequently interface with a wide range of unactivated alkenes for C–N bond formation. This protocol exhibits a broad substrate scope and great functional group tolerance, further highlighting the advantages of excited-state PCET as a platform for catalytic radical generation from common organic functional groups.