10. Catalytic C–N Bond-Forming Reactions Enabled by Proton-Coupled Electron Transfer Activation of Amide N–H Bonds
Lucas Q. Nguyen, Robert R. Knowles:
https://pubs.acs.org/doi/abs/10.1021/acscatal.6b00486
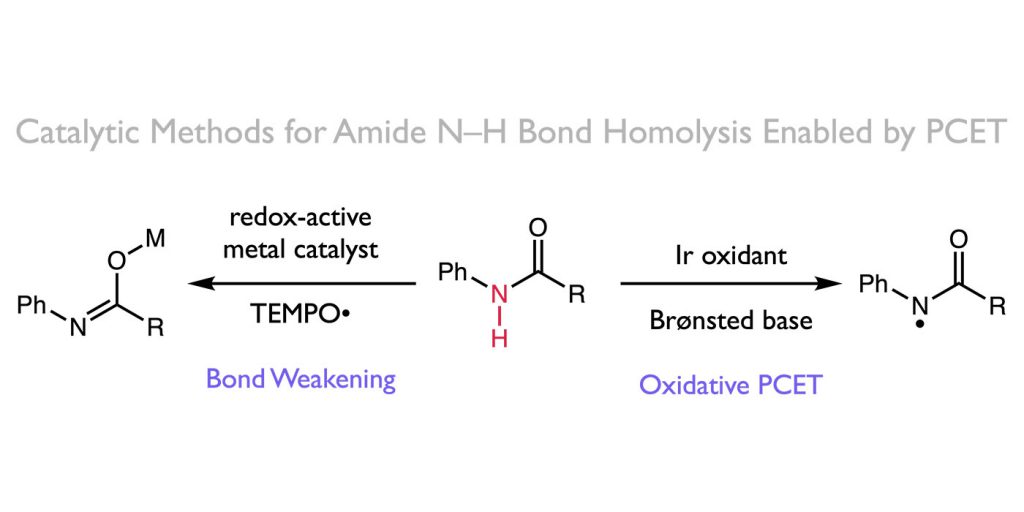
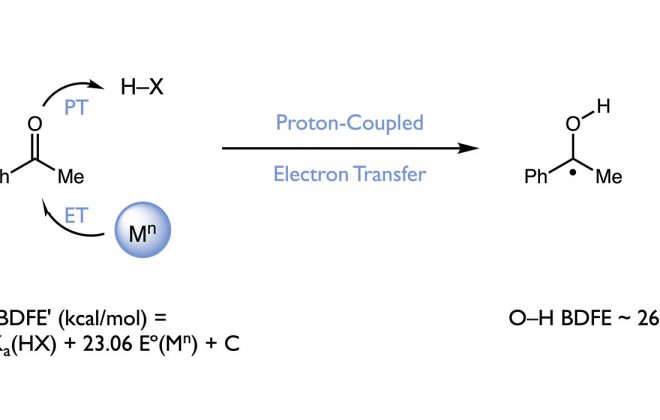
ABSTRACT: Over the past three years, our group has become interested in the ability of proton-coupled electron transfer (PCET) to facilitate direct homolytic bond activations of common organic functional groups that are challenging substrates for conventional hydrogen atom transfer catalysts. This perspective details our efforts to develop oxidative PCET platforms for activating the strong N–H bonds of amides, providing catalytic access to synthetically useful amidyl radicals. We successfully identified compatible combinations of one-electron oxidants and Brønsted bases that, although unable to activate the amide substrates independently, act concomitantly with the requisite energetics to selectively homolyze the N–H bond via concerted PCET. The resulting amidyls were utilized in the development of new catalytic protocols for alkene carboamination and hydroamidation. We also highlight our efforts to develop a PCET-based bond-weakening protocol for the catalytic conjugate aminations using amide substrates. In this work, coordination to a Ti(III) catalyst significantly decreases the strength of a ligated amide N–H bond, enabling a facile PCET event to occur with the weak H atom acceptor TEMPO. Although this discussion focuses on amide activation, we anticipate that the design parameters presented here are general and should provide a framework for the development of PCET catalyst systems for other challenging homolytic bond activations as well.