20. A Redox Strategy for Light-Driven, Out-of-Equilibrium Isomerizations and Application to Catalytic C–C Bond Cleavage Reactions
Eisuke Ota, Huaiju Wang, Nils Lennart Frye, Robert R. Knowles:
https://pubs.acs.org/doi/10.1021/jacs.8b12552
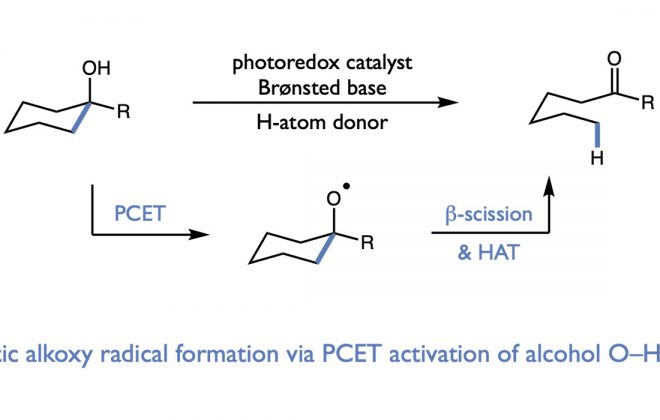
ABSTRACT: We report a general protocol for the light-driven isomerization of cyclic aliphatic alcohols to linear carbonyl compounds. These reactions proceed via proton-coupled electron-transfer activation of alcohol O–H bonds followed by subsequent C–C β-scission of the resulting alkoxy radical intermediates. In many cases, these redox-neutral isomerizations proceed in opposition to a significant energetic gradient, yielding products that are less thermodynamically stable than the starting materials. A mechanism is presented to rationalize this out-of-equilibrium behavior that may serve as a model for the design of other contrathermodynamic transformations driven by excited-state redox events.