14. Catalytic Ring-Opening of Cyclic Alcohols Enabled by PCET Activation of Strong O–H Bonds
Hatice G. Yayla, Huaiju Wang, Kyle T. Tarantino, Hudson S. Orbe, Robert R. Knowles:
https://pubs.acs.org/doi/full/10.1021/jacs.6b06517
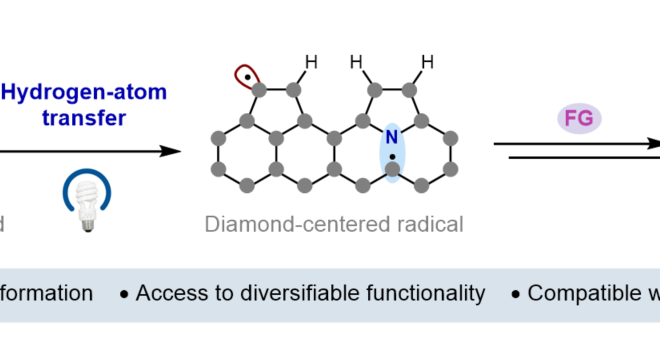
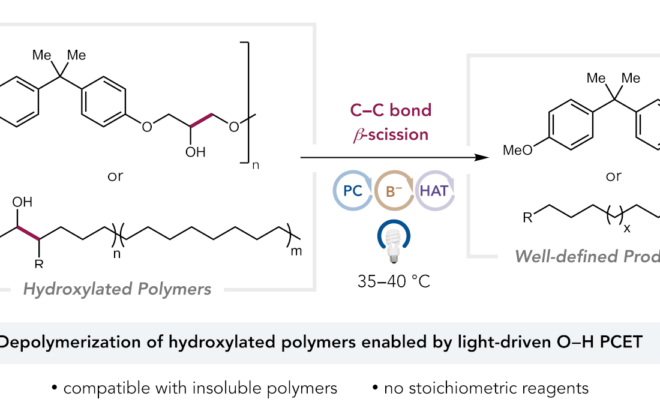
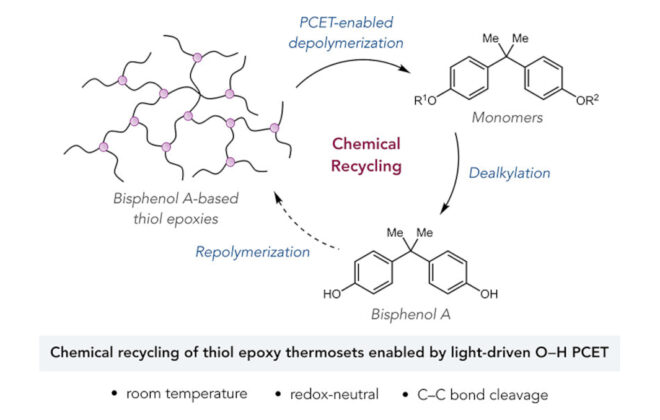
ABSTRACT: We report a new photocatalytic protocol for the redox-neutral isomerization of cyclic alcohols to linear ketones via C–C bond scission. Mechanistic studies demonstrate that key alkoxy radical intermediates in this reaction are generated via the direct homolytic activation of alcohol O–H bonds in an unusual intramolecular PCET process, wherein the electron travels to a proximal radical cation in concert with proton transfer to a weak Brønsted base. Effective bond strength considerations are shown to accurately forecast the feasibility of alkoxy radical generation with a given oxidant/base pair.
Categories