26. Catalytic Ring Expansions of Cyclic Alcohols Enabled by Proton-Coupled Electron Transfer
Kuo Zhao, Kenji Yamashita, Joseph E. Carpenter, Trevor C. Sherwood, William R. Ewing, Peter T. W. Cheng, Robert R. Knowles:
https://pubs.acs.org/doi/full/10.1021/jacs.9b03973
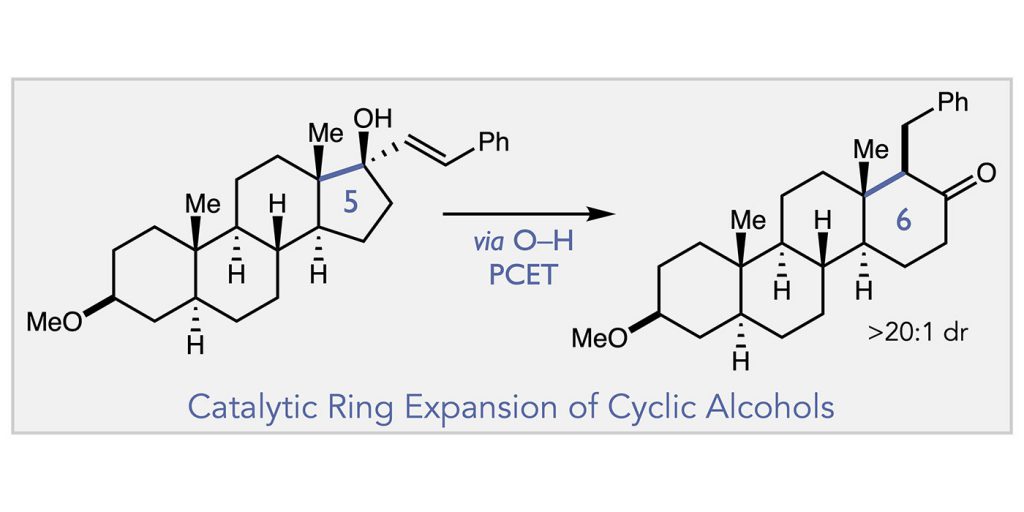
ABSTRACT: We report here a catalytic method for the modular ring expansion of cyclic aliphatic alcohols. In this work, proton-coupled electron transfer activation of an allylic alcohol substrate affords an alkoxy radical intermediate that undergoes subsequent C–C bond cleavage to furnish an enone and a tethered alkyl radical. Recombination of this alkyl radical with the revealed olefin acceptor in turn produces a ring-expanded ketone product. The regioselectivity of this C–C bond-forming event can be reliably controlled via substituents on the olefin substrate, providing a means to convert a simple N-membered ring substrate to either n+1 or n+2 ring adducts in a selective fashion.